
Science in general and scientific findings in particular need to be analyzed according to their socio-historical context of emergence. This chain of innovation becomes more relevant when the framework of these milestones is formed by a global pandemic that gave applied science an unusual speed.
After two years of the world living with the SARS-CoV-2 coronavirus, science analyzes and perfects the various treatments that were put on the table to deal with COVID-19, which has already caused more than 493 million infections and more than 6 million deaths, and which still resists reconverted behind new variants.
A brand-new study published in The New England Journal of Medicine (NEJM) strongly reignited the debate on the plasma intervention of convalescents in people infected with COVID. This scientific journal - considered the most important in the world - came to the same conclusion as the Argentine pediatrician and infectiologist Fernando Polack and his teams, in 2020, when he investigated the use of this early plasma therapy in Argentina, without vaccines yet available, or approved, to control the lethality and high contagiousness demonstrated by the new virus.
Research conducted in more than a thousand cases conducted by the Bloomberg School of Public Health at Johns Hopkins University and the Mayo Clinic, among other medical-scientific institutions, evaluated the efficacy of convalescent plasma in patients in early stages of the disease, and without factors or health comorbidities, and demonstrated that it is an effective and safe option as an early outpatient treatment for the disease.
He concluded that hyperimmune plasma would reduce the risk of hospitalization by half if administered within 9 days after the onset of COVID-19 symptoms, and cited and gathered studies done by Polack and teams as part of the evidence.
During the course of his research with plasma in 2020 - at the beginning of the pandemic - Polack always maintained that , “because of how antibodies work in the body, the application of plasma has a better theoretical chance of working at the beginning of the disease. Because if you get plasma with antibodies and lend them to a newly infected one, they should immobilize the virus.”
How, then, in the current pandemic time effectively controlled by COVID vaccines with a safe, available and diverse health action strategy (across multiple platforms) - and despite the fact that pockets of inequity still exist in the world - this study that rescues the use of plasma hyperimmune as an option against COVID?
Infobae spoke with the infectiologist and pediatrician Fernando Polack to analyze the work published in the NEJM, in his as a leading researcher in the study of convalescent plasma carried out in Argentina.
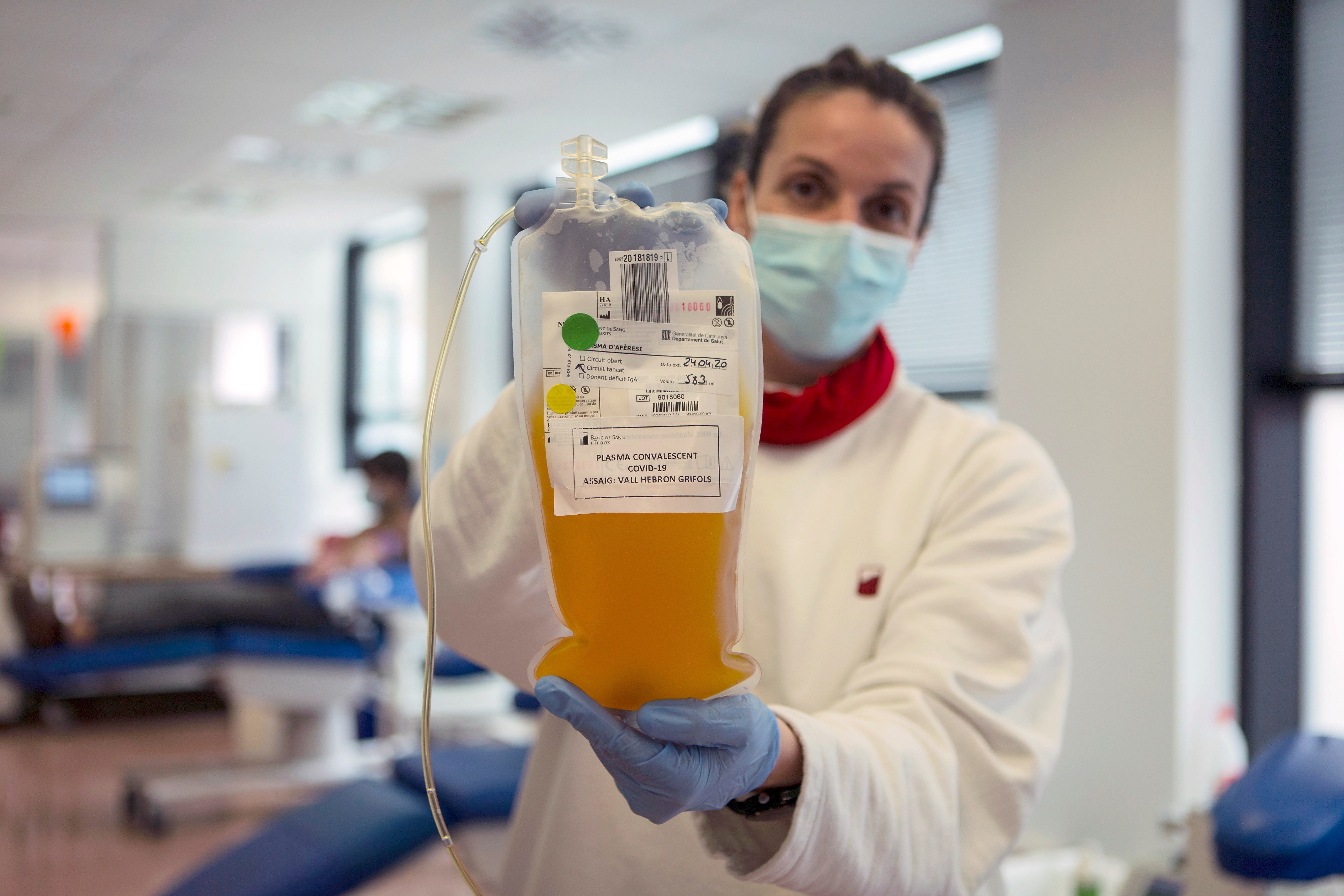
—Plasma treatment of convalescents to slow the spread of COVID-19 played a very important role at the beginning of the pandemic, and today -two years later- and with a powerful portfolio of vaccines against COVID approved - and from different platforms - for the whole world, this study published in the NEJM that claims serum appears hyperimmune of convalescents. What is the place of this intervention today in the face of COVID?
—Fernando Polack: There are essentially two ways to deal with diseases in order to solve them: one is to prevent it and the other is to treat it once it occurs. Obviously, in order to prevent a disease, we must act as early as possible and to treat it within the framework of the same logic as well. In general, whenever you can anticipate the progress of a problem, you are better positioned than when you are solving something that has already happened and that has ramifications that start to deviate from the original problem that caused it.
In COVID, that happens; vaccines, and we researchers know this from experience, what they do is slow the progression of infection and stop it before it becomes a disease or a serious illness. But the dilemma is once the disease is installed how far you can stop it and where you already have to start dealing with the consequences that go beyond the infection itself.
Plasma like monoclonal antibodies should have always been used very early, especially from the perspective of how they work; since they do it exactly the same as how vaccines work.
The difference is that vaccines are antibodies that are generated inside the body to block the progress of the virus and are generated before infection occurs, because because it takes a couple of weeks for those antibodies to be generated there would be no material time for the vaccines to work if they were given after infection, and plasma shortens those times by being administered as soon as possible once the infection occurred. That is the central difference.

—Fernando Polack: Monoclonal antibodies one could say in a very simplified view of things, that they are a kind of “synthetic plasma”, of antibodies made outside the body that are administered with an injection.
Several monoclonal antibodies have worked with the same logic with which we did the initial plasma test that we presented two years ago, and it was obvious that due to a biological matter, the plasma used intelligently early had to have the same result. The study done in Argentina was well thought out and well designed, like the one we did with the province of Buenos Aires, the City of Buenos Aires, PAMI, Swiss Medical, OSDE, OSECAC and many other actors from the Infant Foundation; and now this study by the National Institute of Health of the United States has it and demonstrates this study by the National Institute of Health of the United States with the American universities - Hopkins and Mayo, among others - throughout the country with more than a thousand volunteers.
What do you rescue from this study published in the NEJM that weighs plasma therapy of convalescents at an early stage of the disease, specifically within nine days after the positive test? With the passage of time, do you also think that plasma is currently underused among the treatment options against COVID?
—Polack: What makes me happy about this study published in the NEJM is that it confirms that plasma makes sense and is useful, always in science it is necessary to confirm by more than one group the findings that science undertook in a well-thought-out and well-executed way. Another dilemma of the pandemic was that as everyone started looking for solutions, solutions were sometimes evaluated without a thorough understanding of how the variables that would determine the success of those solutions behaved.
In Argentina, the studies carried out in this regard with plasma were fairly well thought out and well designed. And nothing that we and other groups said at the beginning of the pandemic, ceased to be true two years later. And that's really interesting and important. Well-thought out, well-designed, well-armed science provides solutions. Whether it's vaccines, antibody interventions or anything that has a sound biological logic. At the end of these stories there is no chance, you have to study, work and think.
Plasma today, in this context of the pandemic, is of almost zero or very little use. But at the time we submitted the data, December 2020, it was very useful. Because these findings did not change and the possibility of reducing by almost half or perhaps a third the development of severe COVID-19 disease in older people is a very valuable tool.

—Polack: Perhaps the complexity of its use (of plasma), perhaps a lesser understanding in many parts of the world of what intervention meant, that we did not invent it, nor was it yesterday, it has existed for a century, meant that everything that could have been used is not used to be used.
And then the luck of having been so successful with vaccines that it does make any other preventive strategy really unnecessary. But any intervention against a virus focuses on what I said at the beginning: the early time, when you only have to deal with blocking the virus and not too late when you have to deal with all the consequences that the virus had on the patient's health.
The reference is worth it: it's like grabbing a horse before it enters a bazaar - and breaks everything - or going to look for it once it ran through the bazaar for twenty minutes and there is hardly any healthy dish left. That is essentially the central idea.
And from the point of view of the teams of multidisciplinary researchers in Argentina, the effort we made was enormous. An effort of many groups of doctors, researchers, also society, and teams involved in public health. And it's very good and comforting from the team's point of view to reaffirm that the effort made sense and that what we were saying was right because that's what we're working for.
Our commitment was to seek at that time a solution for Argentina to a problem that had no solution in view. And that then obviously vaccines made those strategies much less necessary.
— How do you imagine the future of convalescent plasma therapy against this and other viruses? Should it always be at hand? Don't you think that an agency like the World Health Organization (WHO) came forward in December 2021 by advising against the use of convalescent plasma in patients with COVID?
—Polack: Today the plasma of convalescents is not needed. That is, the important idea would be that you should not use plasma, you have to get vaccinated. The solution is the vaccine.
But for a long time when there was no vaccine this (convalescent plasma) was an intermediate solution that worked. And we have to keep it in mind because if we ever face another germ, this solution didn't work for the first time with COVID, it worked with more than ten different diseases in the past. There was no reason why I shouldn't do it with this one.
Plasma today does not have a place in the therapeutic strategy against COVID; but it shows that 16 or 18 months ago when Argentine researchers, and a lot of groups of doctors, social actors and public health actors answered what we could use as a bridge until vaccines arrived we came to two conclusions: late plasma is useless in severe patients and plasma in mild patients is a very valuable tool until there is a better solution called vaccine.
Science made in Argentina
Polack also spearheaded the world's largest Phase III trial to test the efficacy and safety of the genetic platform COVID vaccine -Messenger RNA- produced by the scientific binomial Pfizer-Biontech, made at the Military Hospital of the City of Buenos Aires. The results - known in 2020 - were also published in the prestigious scientific journal NEJM.
Polack is considered one of the leading international experts in respiratory viral diseases and heads the Infant Foundation, which is currently working together with a group of multidisciplinary scientists on an international project that seeks to achieve a triple viral vaccine in adults - it will be a trial of more or less a thousand people and currently is Phase II. “We are studying the goal of migrating to a vaccine that is the triple viral vaccine for adults, which would be against coronavirus, influenza and respiratory syncytial virus (RSV),” the infectiologist told Infobae.
The study conducted in Argentina, in 2020, by the Infant Foundation chaired by Polack showed evidence on the early use of convalescent plasma in adults over 65 years of age with mild COVID-19: which demonstrated 61% effectiveness in preventing the coronavirus from developing in a serious illness and evolves only, in the words of the specialist, into “a bad cold”
The study was carried out in collaboration with the Ministry of Health of the Province of Buenos Aires at the facilities of the public hospitals San Juan de Dios, Simplemente Evita, Dr. Carlos Bocalandro and Evita Pueblo. In the capital, the Central Military Hospital, the Los Arcos Sanatorium, CEMIC, the Ministry of Health of the City of Buenos Aires, the Social Work of Commercial Employees (OSECAC) and the Finochietto Sanatorium participated.
For Argentines, plasma already had a prominent place in the history of local science due to its good performance in treating the Argentine hemorrhagic fever epidemic that hit the country 70 years ago. Thus, the treatment of immune plasma of convalescents was able to significantly reduce lethality thanks to the outstanding work of Dr. Julio Maiztegui at that time.
The National Institute of Human Viral Diseases (INEVH) located in the city of Pergamino, Buenos Aires province, which stands out for its work on hantaviruses, dengue, yellow fever and other arboviruses, has positioned it as a national and regional reference center for laboratory diagnosis of these diseases, and for years now It bears the name of the remembered “Dr. Julio Maiztegui”.
Conclusions and the harshness of WHO
The study's lead co-author, David Sullivan, MD, professor of molecular microbiology and immunology at Johns Hopkins Bloomberg School of Public Health, was forceful about the role of convalescent plasma in times of COVID, “as the changing, often unpredictable, landscape of the COVID-19 pandemic calls for multiple treatment options, especially in low- and middle-income countries where first-line therapies such as vaccines and monoclonal antibodies may not be readily available, our study provides strong evidence that antibody-rich convalescent plasma should be part of the ambulatory arsenal,” he said.
The study published in NEJM concluded, “that high-titer (antibody-rich) COVID convalescent plasma, when administered to outpatients for COVID-19 within 9 days after testing positive, reduced the need for hospitalization for more than half of outpatients predominantly unvaccinated.
The World Health Organization (WHO) was harsh on plasma as available therapy against COVID: in early December 2021 it strongly pronounced itself against plasma treatment of convalescent people to treat cases of COVID-19, whether moderate, severe or severe. According to the international organization, the research carried out showed that it does not increase the likelihood of survival, nor does it reduce the need to use respirators.
It is based on analyzing 16 scientific studies involving 16,236 patients with COVID-19 and concluded that plasma treatment is also very expensive and difficult to administer. It also noted a number of practical problems, such as the need to identify and test donors, as well as difficulties in collecting, storing and using plasma, all of which represent additional limitations to making it a viable treatment. The only case in which WHO leaves open the possibility of its use is in the case of a randomized controlled trial.
The U.S. Food and Drug Administration (FDA) currently authorizes convalescent hyperimmune plasma as a treatment option for outpatients with immunocompromised diseases or those receiving immunocompromised drugs, and for all patients hospitalized with early stage COVID-19.
KEEP READING:
Últimas Noticias
Debanhi Escobar: they secured the motel where she was found lifeless in a cistern

The oldest person in the world died at the age of 119

Macabre find in CDMX: they left a body bagged and tied in a taxi
The eagles of America will face Manchester City in a duel of legends. Here are the details

Why is it good to bring dogs out to know the world when they are puppies



