
The pharmaceutical company Moderna today announced the provisional results of its covid-19 vaccine for children under 6 years of age. The company said that two 25-microgram doses of its Covid-19 vaccine for children aged 6 months to 5 years provided an immune response similar to two 100-microgram doses for adults aged 18 to 25, indicating that the benefit conferred on young adults is also conferred on young children.
The results were obtained from the analysis based on a group of 6,900 children aged 6 months to 5 years.
Most adverse reactions were mild or moderate and were more frequent after the second injection. The company noted that no deaths or cases of myocarditis or pericarditis were reported. Myocarditis is inflammation of the heart muscle, and pericarditis is inflammation of the lining of the heart.
The two doses of the vaccine are given to children 28 days apart. The data showed “a strong neutralizing antibody response” and “a favorable safety profile”, according to the pharmaceutical company's statement released today.
In view of these good results, Moderna said it will ask the US Food and Drug Administration (FDA) to authorize the use of the pediatric vaccine for children aged 6 months to 5 years in the coming weeks.
Moderna CEO Stéphane Bancel anticipated that “given the need for a COVID-19 vaccine in infants and young children, we are working with the US FDA and regulators around the world to send these results as soon as possible. We believe that these latest results will be good news for parents of children under 6.”

Less effective against Ómicron
The vaccine was not as effective in preventing COVID-19 infections caused by the Ómicron variant, which predominated in the United States and the world during the study. For children aged 6 months to 1 year, the effectiveness against Omicron was 43.7% and for children aged 2 to 5 years, 37.5%. Moderna explained that the lower efficacy was still statistically significant and consistent with the performance of adults vaccinated with the Ómicron variant.
The US pharmaceutical company is evaluating the potential of a booster vaccine for all children 6 months and older, which would target the ancestral version of the coronavirus, first detected in Wuhan, as well as the Ómicron variant.
Moderna also announced that it has already started a filing with the FDA for the authorization of emergency use of the company's Covid-19 vaccine for children aged 6 to 11 years. Children that age would receive two shots of a larger 50 microgram version of the vaccine.
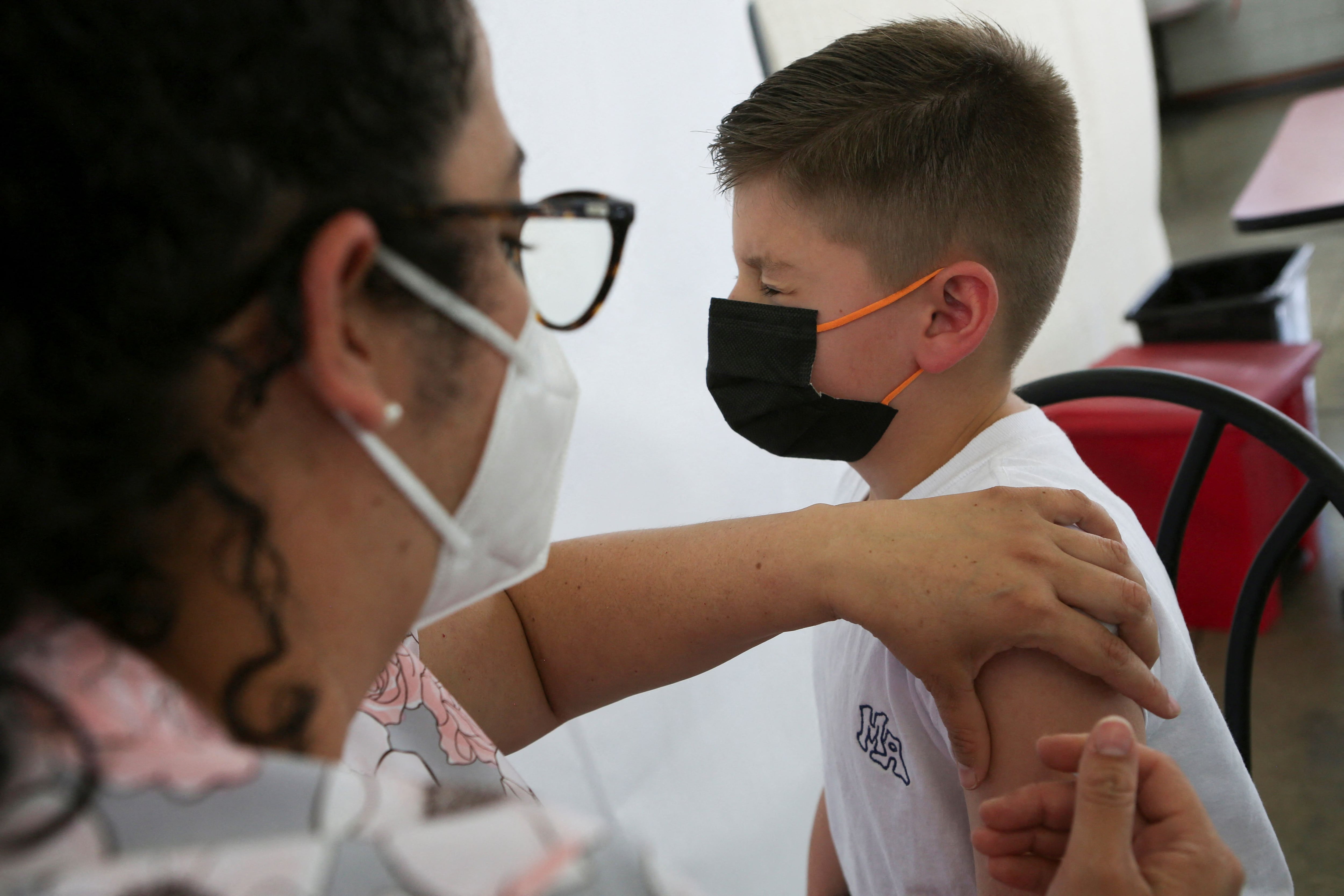
The company noted that it provided the FDA with additional follow-up data on its vaccine for children aged 12 to 17 years. Children that age would receive two shots of a larger 100 microgram version of the vaccine.
Last month, the FDA postponed a meeting of its vaccine advisors to consider the Pfizer/BioNTech COVID-19 vaccine for children under 5 years of age and requested additional data on third doses. The companies said they expect the data to be ready in early April.
KEEP READING:
Últimas Noticias
Debanhi Escobar: they secured the motel where she was found lifeless in a cistern
Members of the Specialized Prosecutor's Office in Nuevo León secured the Nueva Castilla Motel as part of the investigations into the case

The oldest person in the world died at the age of 119
Kane Tanaka lived in Japan. She was born six months earlier than George Orwell, the same year that the Wright brothers first flew, and Marie Curie became the first woman to win a Nobel Prize

Macabre find in CDMX: they left a body bagged and tied in a taxi
The body was left in the back seats of the car. It was covered with black bags and tied with industrial tape
The eagles of America will face Manchester City in a duel of legends. Here are the details
The top Mexican football champion will play a match with Pep Guardiola's squad in the Lone Star Cup

Why is it good to bring dogs out to know the world when they are puppies
A so-called protection against the spread of diseases threatens the integral development of dogs




