
As happened months ago in several countries around the world, on January 4, the National Administration of Drugs, Food and Medical Technology (ANMAT) approved individual use of four self-evaluation tests based on the detection of the SARS-CoV-2 virus.
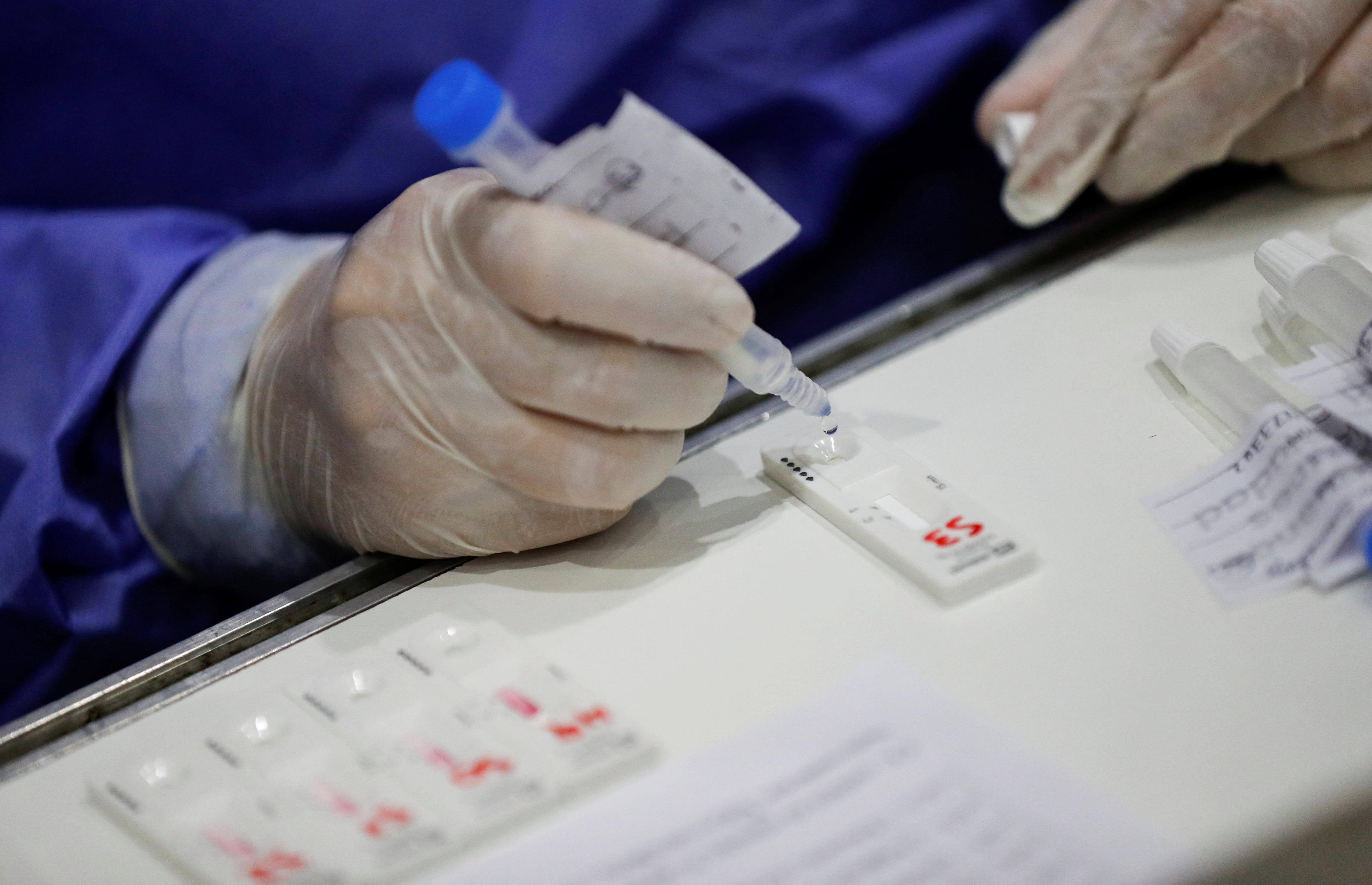
The approved Panbio COVID-19 Antigen Self-Test, SARS-CoV-2 Antigen Self Test Nasal, SARS-CoV-2 Antigen Rapid Test (COVID-19 Ag), and WL Check SARS-CoV-2 Ag Self Testing products belong to Abbott, Roche, Vyam Group and Wiener laboratories and are sold exclusively in pharmacies. They serve as diagnostic guidance and users must collect the sample themselves based on the instructions indicated by the manufacturers on each product.
“It is important to clarify that these tests provide indicative results, without conclusive diagnostic value, except that the jurisdictions, in agreement with the Ministry of Health of the Nation and based on the epidemiological situation, consider the result as positive. Unlike tests for professional use where the sample is taken at the nasopharyngeal level, in the case of self-evaluation tests it is performed at the nasal level or by saliva, as specified by the manufacturer. In this regard, it is very important that the sampling is carried out correctly and that the test is carried out immediately to avoid erroneous results,” said Anmat on that occasion.
Regarding its effectiveness, they clarified: “It is necessary to bear in mind that if the person has no symptoms or if the viral load is low (which may occur during the initial or final days of infection) SARS-CoV-2 may not be detected by the test, so a negative result does not rule out infection. In order to be followed up, the results must be reported immediately (based on the barcode of each package) after the test has been carried out, and a longer period will be available when it has not been used, either by the individual user or by a reporting officer in the event of a large volume of tests”.
Experts point out that beyond the chosen method, what must be taken into account is the type of test that will be done. No, how is it done. Tests carried out by PCR technology (specifically RT-PCR) are more effective than antigen tests, whether performed with saliva or nasopharyngeal sample. Therefore, health authorities recommend having a PCR test after testing positive for a personal test at home to confirm if they have COVID-19. Experts consider the test to be “a very useful public health tool” to stop the spread of the disease. These tests are widely used at home, in workplaces and schools in much of the world.
Researchers at the University of Liverpool, Harvard University and the University of Bath highlight in a paper in The Lancet, that antigen testing (LFT) works in a very different from polymerase chain reaction (PCR) tests and cannot be compared.
“Antigen testing does not require any additional instruments or equipment, which makes it highly portable and can be used in a wide variety of healthcare settings at so-called point-of-care and screening,” explained to Infobae the infectiologist Eduardo López (MN 37586) head of the Department of Medicine of the Ricardo Gutiérrez Children's Hospital.
This test allows great benefits to emergency departments, which would allow the rapid identification of outbreaks and contain the spread of infections. The rapid antigen test detects the nucleocapsid protein of the virus. This protein is found on the surface of the structure of the virus, so the presence of the virus is found much faster. From there, its result lies in a few minutes. In contrast, the PCR test looks for the presence of genetic material from the virus. However, “the rapid test is not a substitute for PCR, but it is a very good alternative to the need for a rapid diagnosis or when PCR diagnoses are not available,” explains López. He adds: “The Ministry of Health recognizes these methods with diagnostic criteria because of their high specificity when they are positive.”
LFTs detect material from proteins on the surface of the virus and are very likely to test positive when someone is infectious, while PCR tests detect the genetic material of the virus, which may be present for weeks after a person is no longer infectious.
The infectiologist Ricardo Teijeiro, for his part, told Infobae: “Since the beginning of the pandemic there were different qualities of antigen tests. Two years later we can say that current antigen tests that are from recognized laboratories are highly effective and that compared to tests done with PCR. That's why they have proven to be very effective.”
María del Mar Tomas, microbiologist at Hospital A Coruña, researcher at INIBIC and spokesperson for SEIMC argues that “saliva is a very effective sample for the detection of the Ómicron variant if we use PCR technology (specifically RT-PCR) or innovative techniques such as CRISPR-Cas”, although she points out that antigen tests in saliva “are less effective and have a lower sensitivity”. Omicron can be transmitted when it has infected the throat and saliva, before the virus has reached the nose, so passing a swab through the nostrils at the beginning of the infection will not detect it.
In a recent study in the United States, PCR tests with saliva of 29 people infected with Omicron detected the virus an average of three days before the nose samples tested positive for antigen tests, or so-called flow lateral. In general, rapid tests have a lower sensitivity than laboratory-processed PCR tests, which means they produce more false negatives. But if the result is positive, it is almost certainly COVID-19, which makes antigen testing a powerful tool for dealing with the pandemic.
Analysis of variants
COVID tests look for small fragments of the virus. For example, it is common for antigen tests to contain antibodies that attach to proteins, or antigens, that are on the surface of the virus.
But mutations of the virus can change the shape of these proteins and make it difficult for antibodies to adhere, resulting in false negatives. So, in January 2021, researchers at the Center for the Development of Technologies Designed with Microsystems for Point-of-Care in Atlanta, USA, began working with NIH and FDA to evaluate the performance of dozens of products already authorized with the new variants.
Laboratory experiments initially raised concerns about the sensitivity of some antigen tests with the Ómicron variant, but the tests seem to work better in the real world than in the laboratory.
Since the fall, when Joe Biden's government announced plans to provide people with access to home testing, scientists have also been helping to make FDA clearance faster for products that can be manufactured in large volumes, including Siemens and SD Biosensor tests. Scientists in Atlanta and the federal government have created a better and more agile model: a systematic way for an independent party to examine products, ensuring their quality, and expedite the approval of those who pass the exam, so that they reach the hands of consumers more quickly.
This work has been “crucial,” according to Michael Mina, a retired Harvard epidemiologist and current chief scientific officer of eMed, which sells home tests. Typically, test developers have to collect their own data and follow the regulatory process. “It's a new approach that the United States could take in evaluating diagnostic tools,” Mina said.
The Rapid Diagnosis (or RadX) program, which aims to help COVID test developers refine, market and increase the availability of their products. So far it has supported 29 manufacturers who have received 40 FDA authorizations for COVID testing, including seven over-the-counter products; and all have been evaluated by the Atlanta team.
“There is no doubt that the RadX program has accelerated the development of the home diagnostics category,” Sean Parsons, CEO of Ellume, wrote in an email. “The program has driven years of advancement in innovation and development.”
KEEP READING:
Últimas Noticias
Debanhi Escobar: they secured the motel where she was found lifeless in a cistern
Members of the Specialized Prosecutor's Office in Nuevo León secured the Nueva Castilla Motel as part of the investigations into the case

The oldest person in the world died at the age of 119
Kane Tanaka lived in Japan. She was born six months earlier than George Orwell, the same year that the Wright brothers first flew, and Marie Curie became the first woman to win a Nobel Prize

Macabre find in CDMX: they left a body bagged and tied in a taxi
The body was left in the back seats of the car. It was covered with black bags and tied with industrial tape
The eagles of America will face Manchester City in a duel of legends. Here are the details
The top Mexican football champion will play a match with Pep Guardiola's squad in the Lone Star Cup

Why is it good to bring dogs out to know the world when they are puppies
A so-called protection against the spread of diseases threatens the integral development of dogs




